Abstract
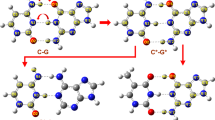
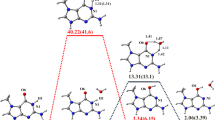
In recent years, there has been increasing interest in damaged DNA and RNA nucleobases. These damaged nucleobases can cause DNA mutation, resulting in various diseases such as cancer. Alkylating agents are mutagenic and carcinogenic in a variety of prokaryotic and eukaryotic organisms. The present study employs density functional theory (DFT/B3LYP) with the 6-311++G(d,p) basis set to investigate the effect of chemical damage in O-alkyl pyrimidines such as O4-methylthymine, O2-methylcytosine and O2-methylthymine. We compared the intrinsic properties, such as proton affinities, gas phase acidities, equilibrium tautomerization and nucleobase pair’s hydrogen bonding properties, of these molecules with those in the normal nucleobases thymine and cytosine. The results are of interest for chemical reasons and also possibly for biological purposes since biological media can be quite non-polar. Furthermore, we found that N1-H of O4-methylthymine is less acidic than N1-H of thymine, suggesting that alkyl DNA glycosylase enzyme cannot discriminate this damaged nucleobase from a normal thymine nucleobase. This result indicates that the conjugated base anion of O4-methylthymine would be a worse leaving group and O4-methylthymine is repaired in genome by demethylation rather than enzyme-catalyzed excision at N1.









Similar content being viewed by others
Notes
Presented at the Spring 2010 meeting of the ACS Division of Physical Chemistry, Multiscale Nanomaterials, Polymer & Bimolecular Dynamics
References
Watson JD, Crick FHC (1953) A structure for deoxyribose nucleic acid. Nature 171:737–738
Brenner S, Jacob F, Meselson M (1961) An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature 190:576–581
Saenger W (1984) Principles of nucleic acid structure. Springer, New York, pp 113–150
Demple B, Harrison L (1994) Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem 63:915–948
Loft S, Poulsen HE (1996) Cancer risk and oxidative DNA damage in man. J Mol Med 74:297–312
Nakabeppu Y, Sakumi K, Sakamoto K, Tsuchimoto D, Tsuzuki T, Nakatsu Y (2006) Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol Chem 387:373–379
Limoli CL, Kaplan MI, Phillips JW, Adair GM, Morgan WF (1997) Differential induction of chromosomal instability by DNA strand-breaking agents. Cancer Res 57:4048–4056
Palmer CM, Serafini DM, Schellhorn HE (1997) Near ultraviolet radiation (UVA and UVB) causes a formamidopyrimidine glycosylase-dependent increase in G to T Transversions. Photochem Photobiol 65:543–549
Singer B, Grunberger D (1983) Molecular biology of mutagens and carcinogens. Plenum, New York, pp 55–79
Beranek DT (1990) Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res 231:11–30
Lo TL (1975) Hard soft acids bases (HSAB) principle in organic chemistry. Chem Rev 75:1–20
Moschel RC, Hudgins WR, Dipple A (1979) Selectivity in nucleoside alkylation and aralkylation in relation to chemical carcinogenesis. J Org Chem 44:3324–3328
Loechler EL (1994) A violation of the swain-scott principle, and not SN1 versus SN2Reaction mechanisms. Explains why carcinogenic alkylating agents can form different proportions of adducts at oxygen versus nitrogen in DNA. Chem Res Toxicol 7:277–280
Lu X, Heilman JM, Blans P, Fishbein JC (2005) The Structure of DNA dictates purine atom site selectivity in alkylation by primary diazonium ions. Chem Res Toxicol 18:1462–1470
Lawley P, Bartsch D, Tomatis L (1976) Screening tests in chemical carcinogenesis. IARC Scientific Publications, Lyon, France, pp 181–208
Singer B, Kroger M, Carrano M (1978) O2- and O4-Alkyl pyrimidine nucleosides: stability of the glycosyl bond and of the alkyl group as a function of pH. Biochemistry 17:1246–1250
Loveless A (1969) Possible relevance of O6-Alkylation of deoxyguanine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature 223:205–207
Coulondre C, Miller JH (1977) Mutagenic specificity in the Lacl gene of Escherichia coli. J Mol Biol 117:577–606
Richardson KK, Richardson FC, Swenberg JA, Skopek TR (1987) DNA base changes and alkylation following in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Proc Natl Acad Sci USA 84:344–348
Abbott PJ, Saffhill R (1979) DNA synthesis with methylated Poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O′-methylguanine. Biochim Biophys Acta 562:51–61
Beranek DT, Heflich RH, Kodell RL, Morris SM, Casciano DA (1983) Correlation between specific DNA-methylation products and mutation induction at the HGPRT locus in Chinese hamster ovary cells. Mutat Res 110:171–180
Loechler EL, Green CL, Essigmann JM (1984) In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci USA 81:6271–6275
Singer B, Sagi J, Kusmierek JT (1983) The vinyl chloride-derived nucleoside, N2,3-ethenoguanosine, is a highly efficient mutagen in transcription. Proc Natl Acad Sci USA 80:4884–4888
Chandra AK, Nguyen MT, Uchimaru T, Zeegers-Huyskens T (1999) Protonation and deprotonation enthalpies of guanine and adenine and implications for the structure and energy of their complexes with water: comparison with uracil, thymine, and cytosine. J Phys Chem A103:8853–8860
Richardson KK, Richardson FC, Swenberg JA, Skopek TR (1986) DNA base changes in alkylation due to methylnitrosourea (MNU) or ethylnitrosourea (ENU) in E. coli. Proc Am Assoc Cancer Res 27:95–95
Singer B, Spengler S, Bodell WJ (1981) Tissue-dependent enzyme-mediated repair or removal of O-ethyl pyrimidines and ethyl purines in carcinogen-treated rats. Carcinogenesis 2:1069–1073
Spartan ‘06V102’ Wavefunction, Irvine, CA
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti conelation energy formula into a functional of the electron density. Phys Rev B 37:785–789
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094
Carpenter JE, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by “different hybrids for different spins” natural bond orbital procedure. J Mol Struct 169:41–62
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Alkorta I, Elguero J, Foces-Foces C (1996) Dihydrogen bonds (A–HH–B). Chem Commun 14:1633–1634
Alkorta I, Rozas I, Elguero J (1998) Bond length-electron density relationships: from covalent bonds to hydrogen bond interactions. Struct Chem 9:243–247
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Bader RFW (2002) AIM2000 program package, Ver. 2.0. McMaster University, Hamilton
Kosenkov D, Kholod Y, Gorb L, Shishkin O, Hovorun DM, Mons M, Leszczynski J (2009) Ab initio kinetic simulation of gas-phase experiments: tautomerization of cytosine and guanine. J Phys Chem B 113:6140–6150
Mons M, Piuzzi F, Dimicoli I, Gorb L, Leszczynski J (2006) Picosecond X-ray absorption spectroscopy of a photoinduced Iron(II) spin crossover reaction in solution. J Phys Chem A 110:38–44
Gorb L, Kaczmare A, Gorb A, Sadle AJ, Leszczynski J (2005) Thermodynamics and kinetics of intramolecular proton transfer in guanine. Post Hartree–Fock Study. J Phys Chem B 109:13770–13776
Katritzky AR, Karelson M (1991) AM1 calculations of reaction field effects on the tautomeric equilibria of nucleic acid pyrimidine and purine bases and their 1-Methyl analogs. J Am Chem Soc 113:1561–1566
Ha TK, Gunthard HH (1993) Quantum chemical investigation of the structure and stability of all geometric isomers and conformers of all tautomeric forms of thymine. J Am Chem Soc 115:11939–11950
Ha TK, Keller HJ, Gunde R, Gunthard HH (1999) Energy increment method based on quantum chemical results: a general recipe for approximative prediction of isomerization and tautomerization energies of pyrimidine and purine nucleic acid bases and related compounds. J Phys Chem A 103:6612–6623
Nir E, Janzen C, Imhof P, Kleinermanns K, De Vries MS (2001) Guanine tautomerism revealed by UV–UV and IR–UV hole burning spectroscopy. J Chem Phys 115:4604–4611
Nir E, Muller M, Grace LI, De Vries MS (2002) REMPI spectroscopy of cytosine. Chem Phys Lett 355:59–64
Rejnek J, Hanus M, Labelac M, Ryjacek F, Hobza P (2005) Correlated ab initio Study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment and in aqueous solution. Part 4. Uracil and thymine. Phys Chem Chem Phys 7:2006–2017
Plutzer C, Kleinermanns K (2002) Tautomers and electronic states of jet-cooled adenine investigated by double resonance spectroscopy. Phys Chem Chem Phys 4:4877–4882
Shukla MK, Leszczynski J (2006) Spectral origins and ionization potentials of guanine tautomers: theoretical elucidation of experimental findings. Chem Phys Lett 429:261–265
Kosenkov D, Gorb L, Shishkin O, Sponer V, Leszczynski J (2008) Tautomeric equilibrium, stability, and hydrogen bonding in 2′-Deoxyguanosine monophosphate complexed with Mg2+. J Phys Chem B 112:150–157
Mons M, Dimicoli I, Piuzzi F, Tardivel B, Elhanine M (2002) Tautomerism of the DNA base guanine and its methylated derivatives as studied by gas-phase infrared and ultraviolet spectroscopy. J Phys Chem A 106:5088–5094
Trygubenko A, Bogdan TV, Rueda M, Orozco M, Luque FJ, Sponer J, Slavıcek P, Hobza P (2002) Hydrated hydronium: a cluster model of the solvated electron? Phys Chem Chem Phys 4:4192
Kryachko EK, Nguyen MT, Zeegers-Huyskens T (2001) Theoretical study of tautomeric forms of uracil. 1. Relative order of stabilities and their relation to proton affinities and deprotonation enthalpies. J Phys Chem A 105:1288–1295
Kryachko ES, Nguyen MT, Zeegers-Huyskens T (2001) Theoretical study of uracil tautomers. 2. Interaction with water. J Phys Chem A 105:1934–1943
Fogarasi G (2002) Relative stabilities of three low-energy tautomers of cytosine: a coupled cluster electron correlation study. J Phys Chem A 106:1381–1390
Piacenza M, Grimme S (2004) Systematic quantum chemical study of DNA-base tautomers. J Comput Chem 25:83–99
Yang Z, Rodgers MT (2004) Theoretical studies of the unimolecular and bimolecular tautomerization of cytosine. Phys Chem Chem Phys 6:2749–2757
Tsolakidis A, Kaxiras E (2005) A TDDFT study of the optical response of DNA bases, base pairs, and their tautomers in the gas phase. J Phys Chem A 109:2373–2380
Civcir PU (2000) A theoretical study of tautomerism of cytosine, thymine, uracil and their 1-methyl analogues in the gas and aqueous phases using AM1 and PM3. J Mol Struct (THEOCHEM) 532:157–169
Fogarasi G, Szalay PG (2002) The interaction between cytosine tautomers and water: An MP2 and coupled cluster electron correlation study. Chem Physic Lett 356:383–390
Liu M, Tingting L, Amegayibor FS, Cardoso DS, Fu Y, Lee JK (2008) Gas-phase thermochemical properties of pyrimidine nucleobases. J Org Chem 73:9283–9291
Barker DL, Marsh RE (1964) The crystal structure of cytosine. Acta Crystallogr 17:1581–1587
McClure RJ, Craven BM (1973) New investigation of cytosine and its monohydrate. Acta Crystallogr Sect B 29:1234–1238
Weber HP, Craven BM, McMullan RK (1980) The structure of deuterated cytosine monohydrate at 82 K by neutron diffraction. Acta Crystallogr Sect B 36:645–649
Szczesniak M, Szczepaniak K, Kwiatkowski JS, Kubulat K, Person WB (1988) Matrix isolation infrared studies of nucleic acid constituents. 5. Experimental matrix-isolation and theoretical ab initio SCF molecular orbital studies of the infrared spectra of cytosine monomers. J Am Chem Soc 110:8319–8330
Brown RD, Godfrey PD, McNaughton D, Pierlot AP (1989) Tautomers of cytosine by microwave spectroscopy. J Am Chem Soc 111:2308–2310
Morsy MA, Al-Somali AM, Suwaiyan A (1999) Fluorescence of thymine tautomers at room temperature in aqueous solutions. J Phys Chem B 103:11205–11210
Fan J, Shang Zh, Liang J, Liu X, Jin H (2010) Systematic theoretical investigations on the tautomers of thymine in gas phase and solution. J Mol Struct (Theochem) 939:106–111
Huang Y, Kenttamaa H (2003) Theoretical estimations of the 298 K gas-phase acidities of the pyrimidine-based nucleobases uracil, thymine, and cytosine. J Phys Chem A 107:4893–4897
Podolyan Y, Gorb L, Leszczynski J (2000) Protonation of nucleic acid bases. a comprehensive Post-Hartree–Fock study of the energetics and proton affinities. J Phys Chem A 104:7346–7352
Chen ECM, Chen ES (2000) Negative ion mass spectra, electron affinities, gas phase acidities, bond dissociation energies, and negative ion states of cytosine and thymine. J Phys Chem B 104:7835–7844
Chen ECM, Herder C, Chen ES (2006) The experimental and theoretical gas phase acidities of adenine, guanine, cytosine, uracil, thymine and halouracils. J Mol Struct 798:126–133
Russo N, Toscano M, Grand A, Jolibois F (1998) Protonation of thymine, cytosine, adenine, and guanine dna nucleic acid bases: theoretical investigation into the framework of density functional theory. J Comput Chem 19:989–1000
Colominas C, Luque FJ, Orozko M (1996) Tautomerism and protonation of guanine and cytosine. Implications in the formation of hydrogen-bonded complexes. J Am Chem Soc 118:6811–6821
Sponer J, Leszczynski J, Hobza P (1996) Structures and energies of hydrogen-bonded DNA base pairs. A Nonempirical Study with inclusion of electron correlation. J Phys Chem 100:1965–1974
Guerra CF, Bickelhaupt FM, Snijders JG, Baerends EJ (1999) The Nature of the hydrogen bond in dna base pairs: the role of charge transfer and resonance assistance. Chem Eur J 5:3581–3594
Meot-Ner M (1979) Ion thermochemistry of low-volatility compounds in the gas phase. Intrinsic basicities and hydrogen-bonded dimers of nitrogen heterocyclics and nucleic bases. J Am Chem Soc 101:2396–2403
Greco F, Liguori A, Sindona G, Uccella N (1990) Gas-phase proton affinity of deoxyribonucleosides and related nucleobases by fast atom bombardment tandem mass spectrometry. J Am Chem Soc 112:9092–9096
NIST Chemistry WebBook, Linstrom PJ, Mallard WG (eds) (2005) NIST standard reference database number 69. National Institute of Standards and Technology, Gaithersburg, http://webbook.nist.gov
Szczesniak M, Leszczynski J, Person WB (1992) Identification of the Imino-oxo form of 1-Methylcytosine. J Am Chem Soc 114:2731–2733
Wilson MS, McCloskey JA (1975) Chemical ionization mass spectrometry of nucleosides. Mechanisms of ion formation and estimations of proton affinity. J Am Chem Soc 97:3436–3444
Yurenko YP, Zhurakivsky RO, Samijlenko SP, Ghomi M, Hovorun DM (2007) The whole of Intramolecular H-bonding in the Isolated DNA Nucleoside Thymidine. AIM Electron Density Topological Study. Chem Phys Lett 447:140–146
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173
Yurenko YP, Zhurakivsky RO, Samijlenko SP, Hovorun DM (2011) Intramolecular CH⋯O Hydrogen bonds in the AI and BI DNA-like conformers of canonical nucleosides and their Watson-Crick Pairs. Quantum chemical and AIM analysis. Biomol Struct Dyn 1:51–56
Parikh SS, Walcher G, Jones GD, Slupphaug G, Krokan HE, Blackburn GM, Tainer JA (2000) Uracil-DNA glycosylase–DNA substrate and product structures: conformational strain promotes catalytic efficiency by coupled stereoelectronic effects. Proc Natl Acad Sci USA 97:5083–5088
Berti PJ, McCann JAB (2006) Toward a detailed understanding of base excision repair enzymes: transition state and mechanistic analyses of N-Glycoside hydrolysis and N-Glycoside transfer. Chem Rev 106:506–555
Tehrani AZ, Torabifard H, Fattahi A (2012) Thermochemical properties of some vinyl chloride-induced DNA lesions: detailed view from NBO & AIM analysis. Struct Chem 23:1987–2001. doi:10.1007/s11224-012-0026-y
Acknowledgment
Support from Sharif University of Technology is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliakbar Tehrani, Z., Fattahi, A. Comparison of gas phase intrinsic properties of cytosine and thymine nucleobases with their O-alkyl adducts: different hydrogen bonding preferences for thymine versus O-alkyl thymine. J Mol Model 19, 2993–3005 (2013). https://doi.org/10.1007/s00894-013-1813-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1813-0